Richard Lewontin's "The Triple Helix": Review and Response
Richard Lewontin's "The Triple Helix" is principally concerned with breaking down two dogmatic principles in biological thinking: that environments determine genotypes, and that genotypes determine phenotypes. Even in introductory textbooks, it is never expressed as unequivocally as this, and in our day-to-day lives we hardly live as if these relationships are so simple. Nonetheless, this simplified model of relations between environment, genetics, and organism, become fixed in our thinking over time, simplified after repeated uncritical usage in the classroom. Frequently they are explained through simple case studies; discussed at length in my high school classroom was the English black peppered moth, Biston betularia, which experienced a rapid and noticeable change in genotypic and phenotypic composition in response to a rapidly changing environment. When discussing genotypes, some of our lessons modeled the experiments conducted on Drosophilia flies, a very common medium of experimentation for developmental and genetic biologists.
These examples are not false, and neither are the basic principles they explain. However, if we want to be good biologists, we must come to fully understand the complex interpenetration of organisms, their genetics, and their environment.
The genes of an organism are a fundamental input in the outcomes of its development, Lewontin admits. The connection between a particular gene and a particular phenotypic outcome, however, is extremely convoluted. Most distinguishing phenotypes are directly influenced by a large number of genes, and indirectly by an even greater number. More importantly, an organism’s environment significantly affects the way genes are expressed. Genetically identical organisms can have significant developmental variation when placed in different environments, or even when placed in the same environment! And some genetic variation, while producing developmental variation in some environmental conditions, does not produce developmental variation in other kinds of environments!
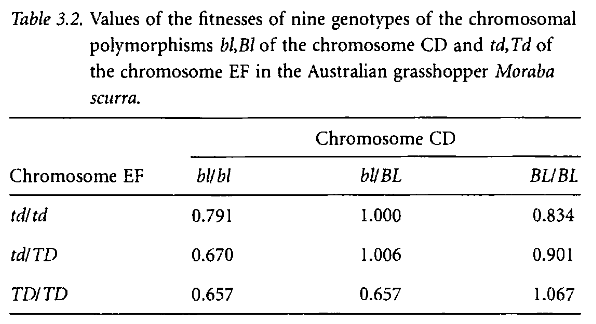
These facts are made even more complex when we consider mechanisms of natural selection. For example, a species may possess a mutation of a particular gene that generally produces a desirable physiological change, improving the fitness of the species. Over enough time, we would expect this variant in the organism to become dominant, resulting in evolution of the species. There may be a second mutation, of a different gene, that also produces a desirable change, also improving the fitness of the species, etc. It is not uncommon, interestingly, for these two desirable mutations to interact negatively, meaning the presence of one or the other is beneficial, while the combination of both is undesirable! This is precisely the situation in Moraba scurra, a grasshopper Lewontin examines to illustrate this point.
The first table illustrates the fitness values (how evolutionary fit) of given combinations of particular genotypes in this grasshopper. A higher value is a higher chance of surviving and passing on those particular combinations, and vice versa. One genotype is not universally preferable; its fitness always depends, in some complex relationship, on the other gene as well.
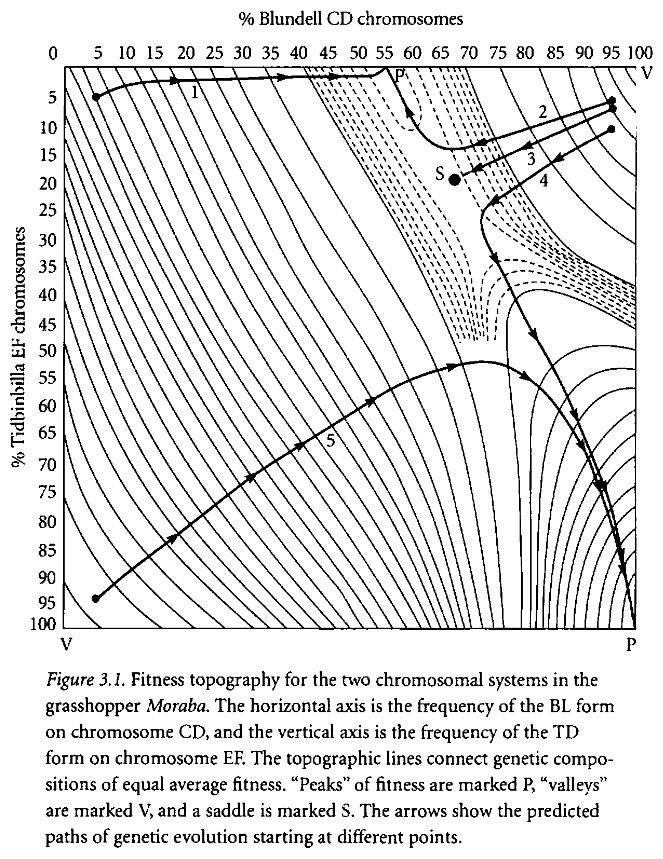
The second table illustrates the same point, with genes represented as percentages of the population rather than as specific combinations. The arrowed lines show the movement of the population average given various starting points; ie, change in genotype distribution over time. If, for example, if a population of grasshoppers began with a genetic makeup mostly possessing the TD/TD and bl/bl genotypes, (the bottom left corner), natural selection will produce first a population mostly consisting of td/TD and bl/BL genotypes, and only then for the more desirable TD/TD and BL/BL genotypes! It is not possible to move linearly from bl/bl to BL/BL, because the intermediate combination has a very low level of fitness. Similarly, given other starting points of genetic variation, it may be impossible to move to the most fit genetic type; this is the situation illustrated by the lines of motion in the top half of the diagram.
One can imagine how much more complicated this situation gets when there are even more kinds of genetic variations at play.
The more tricky relationship is the one between an organism and its environment. Lewontin begins this section by critiquing the concept of an ecological niche. Although a fundamental piece of our vocabulary in the ecological sciences, it does not hold up well to scrutiny. Behind the concept of an ecological niche is a static, unchanging environment, perforated in various places to allow organisms to "fit in". The roles available to play in a given system have already been determined by the environment. What is missing from this picture is the way organisms construct their own environments, and the way every organism is an element in the “environment” of every other organism! The perspective we ordinarily use reduces the organism to simple ecological function, rather than seeing them as unique actors.
In order to more completely understand organisms, then, we must see environments as the “penumbra of external conditions that are relevant to an organism because it has effective interactions with those aspects of the outer world” (pg. 48). Penumbra is an astronomical term that refers to the half-dark, half-lit region at the edge of a shadow cast by an object. What this view entails is that every environment is a zone of ambiguity, which is constructed partially by its organism and partially by the rest of material reality.
This is very easy to see in animals such as beavers, which literally construct their environments by building dams, raising the water table, constructing shelters, and changing the composition of woody plants in the nearby environments, as well as the composition of aquatic and semiaquatic species living in the modulated environments. Other situations may not be so simple.
Here is another example drawn from my own experiences: let us take the oak savanna ecosystems, which are frequently subject to human-induced wildfires. Mature oaks have thick bark which protects them from these wildfires, while many other trees are unable to withstand them. Dead oak leaves are thick, full of tannins (making them difficult to decompose), and persist on the tree for a greater time period. They also curl and are rigid; these all combine to create a humus which is airy, dry, and fuel-rich, which increases the frequency and intensity of fires!
It may be easy enough to say that the encouragement oaks offer to fires is not a result of their “activity”, but is simply determined by their genetics. This is not untrue. But, if we are interested in forming a more sophisticated research program, we might study, for example, what kind of changes in oak development are induced by it being exposed to a wildfire, or other environmental conditions. We might see that the changes the oak undergoes in response to its environment go on to affect the environment itself. We would have a dynamic system of parts always affecting one another, and then recieving their own effects through their surroundings; the causal relationships are circular, not linear.
Lewontin's book is full of more complex arguments and illustrative examples. I have attempted here to reproduce some of the more interesting and fundamental points, but I have hardly expressed the full scope or depth of the work. I would recommend it very strongly to any person studying, or even interested in any kind of biological science. Due to its age, and the frequent changes in these fields, I would not be surprised if some of the particular research discussed is slightly out-of-date; nonetheless, the fundamental problems discussed are still prevalent in so many of my classes today. I hope to make the kind of complex thinking he demonstrates useful in my own studies.
Posted 8-Apr-2023